Medlab Middle East to Reveal Latest Innovations and Technologies in Mobile Labs for COVID-19 Testing
Posted on 14 Jan 2022
The latest technology and innovation in the global fight against COVID-19 and beyond will be on show at the next edition of Medlab Middle East organized by Informa Markets (London, UK), when it returns to the Dubai World Trade Centre from 24-27 January 2022 with mobile molecular testing and diagnosis stations, or ‘laboratories on wheels’, from companies such as Seegene, Inc. (Seoul, Korea) taking centre stage.
Mobile laboratories offer testing and diagnosis on the go, with many having the capacity to conduct upwards of 7,500 tests per day and a turnaround time of just 3.5 hours. The tests cover a variety of pathogens and offer a streamlined, automated workflow, from pre-extraction to data analysis. Typically, a mobile lab features an equipment room and an extraction room and laboratory. Technology within the station allows for automated testing with little supervision while incorporating other equipment such as RT-PCR reagents, consumables, and technical support for diagnostic testing. The other benefit mobile laboratories offer is they can be transported via ship or lorry to almost any place in the world and are fully operational within days of arrival. They can then conduct mass testing in schools, airports, or communities impacted by the pandemic.
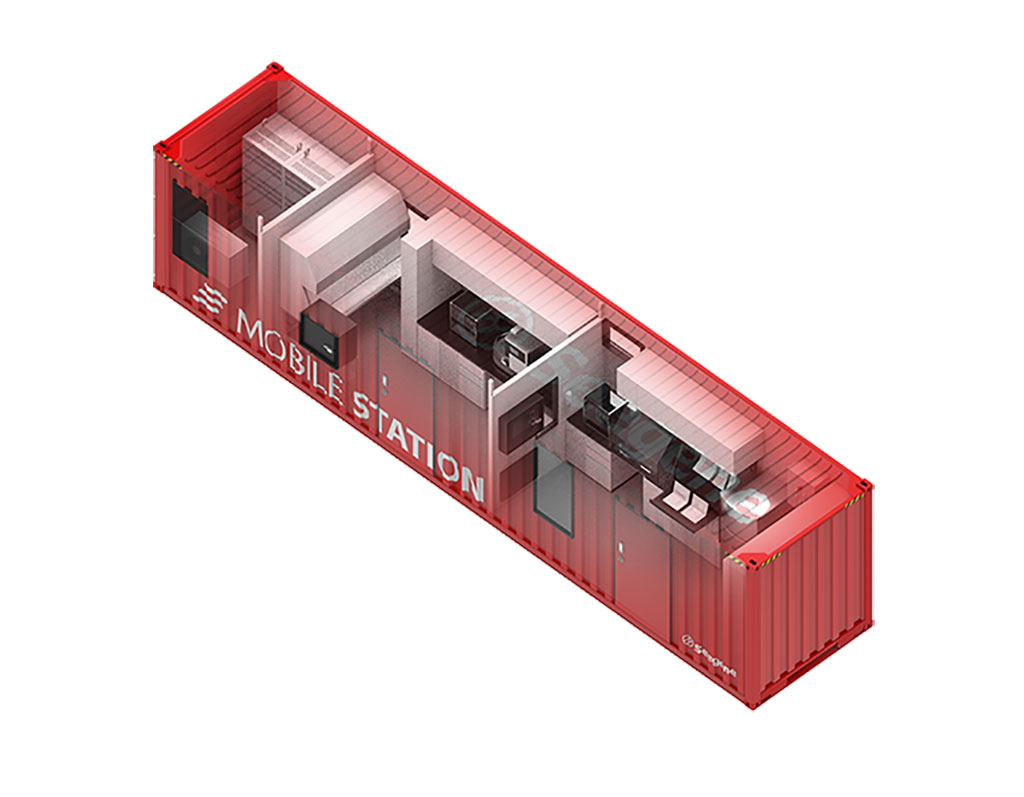
Seegene will be among the companies to showcase its latest innovative products concerning COVID-19 testing during the event. The company recently partnered with Abu Dhabi’s G42 Healthcare to offer mobile laboratory stations across the Middle East and North Africa (MENA) region. In addition to highlighting the latest technology in the fight against COVID-19, the event will also see the return of the Medlab Middle East Congress COVID-19 Updates track. A number of global experts from USA, Europe and GCC will highlight further developments on COVID-19 vaccines and updates on the management of COVID-19 variants. The event will take place under strict protocols and enhanced measures that include 35 guidelines covering all aspects of cleaning and hygiene, social distancing measures, and the use of PPE, screening, and a track and trace in conjunction with local authorities.
“Mobile labs offers the most innovative mobile molecule diagnostic, testing and laboratory services and have had a major impact on the support they have been able to provide the healthcare community in combating COVID-19 infections,” said Tom Coleman, Exhibition Director for Informa Markets who are organizing Medlab Middle East. “As a result of COVID-19, we have seen the laboratory industry fast track a range of new cutting-edge technologies that have had a remarkable impact in the fight against the pandemic. Seegene’s Mobile Station is a prime example of accelerating transformation within the laboratory market, something we will be showcasing during Medlab Middle East in January.”
Related Links:
Seegene, Inc.
Informa Markets













