Mast Group Enters into Multi-Country Distribution Agreement for Nanomix's eLab System
Posted on 08 Nov 2022
Mast Group Limited (Liverpool, UK) and Nanomix (Emeryville, CA, USA) have entered into a multi-region distribution agreement wherein Mast will market and distribute the Nanōmix eLab system in the UK, Germany, France, Ireland and South Africa.
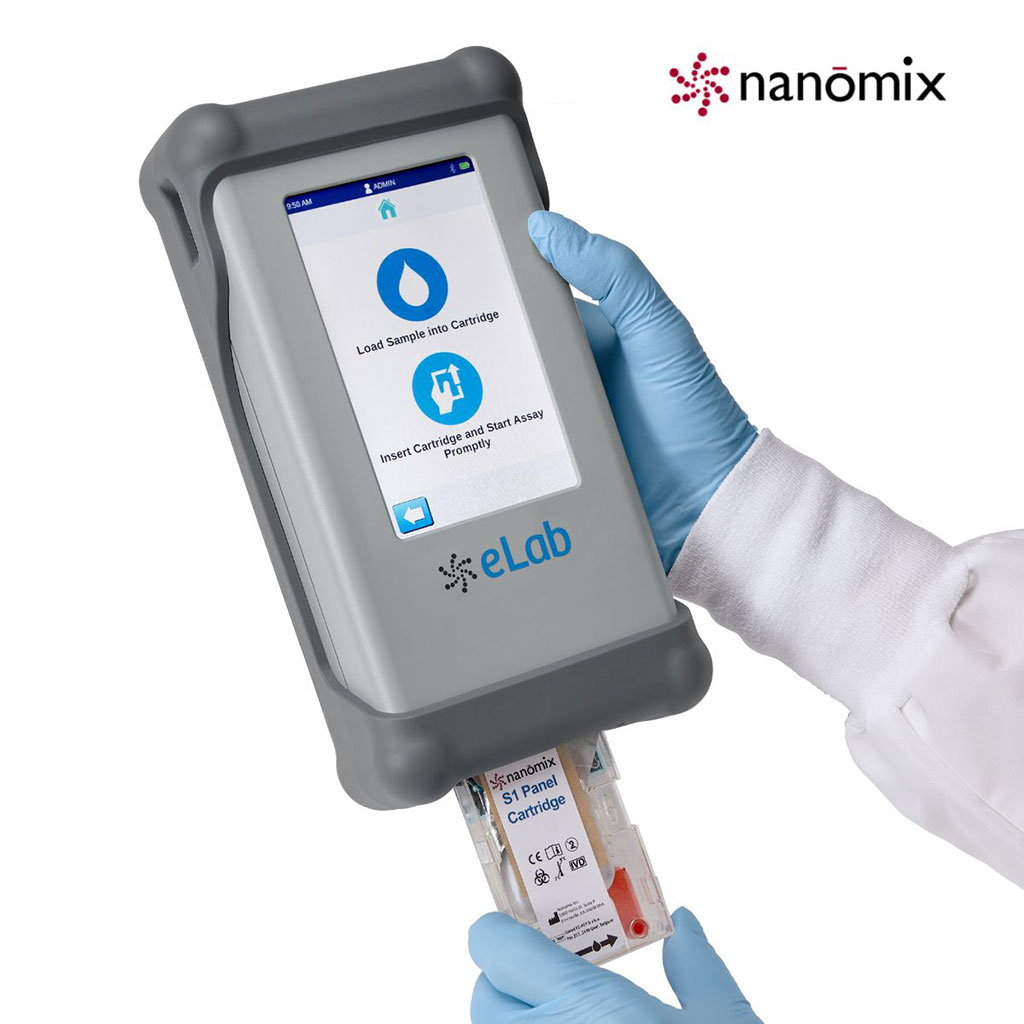
The Nanōmix eLab system is a mobile, hand-held immunoassay and chemistry diagnostic system designed for the needs of rapid point-of-care testing. The system offers a variety of benefits, including results in minutes, lower cost, and portability, while providing accurate, quantitative results comparable in quality to those provided by central lab testing. Furthermore, the S1 Panel Cartridge was developed as an aid in rapidly diagnosing critical infections including sepsis. The panel provides quantitative test results for procalcitonin (PCT), C-reactive protein (CRP) and lactate (LAC) from a single venous whole blood or plasma sample type. The assay runs on the eLab Analyzer with results available in approximately 12 minutes from sample to answer, versus the current diagnostic solutions which can take hours to provide a test result. The S1 Panel assay has received the CE marking in Europe and has UK Medicines and Healthcare products Regulatory Agency (MHRA) registration.
“This new agreement with Mast Group becomes our largest expansion to date within Europe for the Nanomix eLab system,” said Thomas Schlumpberger, Chief Executive Officer of Nanomix. “The Nanomix solution is a unique, breakthrough technology that offers mobile, timely diagnostic capability from a whole blood sample. The eLab system will help expedite sepsis and pneumonia diagnosis leading to more informed treatment decisions, thus improving patient outcomes and hospital clinical collaboration.”
“It has been a pleasure working with The Mast Group to bring this agreement to fruition. Mast has significant experience and presence in this increasingly complex and important area of critical infections, sepsis, and antibiotic stewardship,” said John Hardesky, Chief Commercial Officer of Nanomix. “Their team is experienced and is actively participating in clinical conversations and solutions to improve patient outcomes and impact hospital performance. We are excited to actively align with Mast on an aggressive execution plan to bring our technology to multiple markets throughout Europe.”
“The Mast Group has always been proud of its commitment and ability to offer innovative diagnostic products that help our customers,” added Sandy Daun, Sales and Marketing Director of Mast Group. “We are pleased to begin this long-term, strategic relationship with Nanomix which reinforces that commitment. The platform technology provides the right foundation for assay expansions which will complement the growth areas important to Mast and our customers.”
Related Links:
Mast Group Limited
Nanomix













